-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Welch-Allyn REUSE-12L-2MF - FlexiPort Blood Pressure Cuff; Size-12L Large Adult Long, Reusable, 2-Tubes (8.0 and 13.0 in/20.3 and 33.0 cm), Mated Locking/Subminiature (#5082-182 and #5082-178)
Welch Allyn FlexiPort Blood Pressure Cuff; Size-12L Large Adult Long, Reusable, 2-Tubes (8.0 and 13.0 in/20.3 and 33.0 cm), Mated Lo cking/Subminiature (#5082-182 and #5082-178)
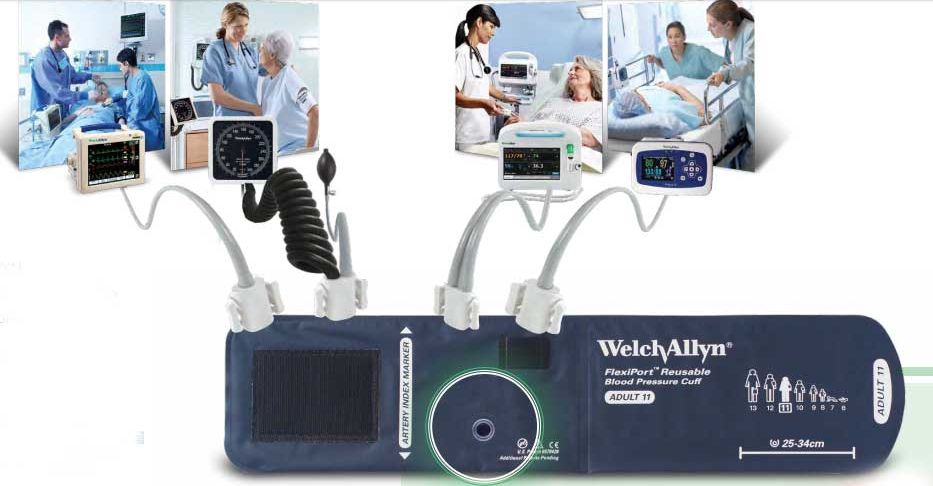
Longlasting, easy to use, and affordable, Welch Allyn cuffs are the top choice for hospitals and clinics around the world. Why? Because Welch Allyn can offer you virtually every type, size, and configuration of cuffs, along with the ability to simplify inventory like no other cuff provider with our innovative FlexiPort connection, which makes every cuff a one- or two-tube cuff.
- Easy one-hand attachment/detachment of tubing from FlexiPort makes changing
- cuffs much faster and easier
- Tested to withstand demanding clinical environments
- 3-year warranty
- Meets all the latest clinical guidelines for proper fit from AAMI and AHA
- Standardising can reduce part numbers by up to 60%
FlexiPort Reusable Cuffs
True standardisation in a snap.

The True Standard in Blood Pressure Cuff Standardization
When it comes to standardizing blood pressure cuffs, Welch Allyn FlexiPort Blood Pressure Cuffs can help hospitals improve financial outcomes and enhance patient safety with options that help streamline workflow and inventory management, and reduce the risk of cross-contamination.
The secret to true cuff standardization is the single-point FlexiPort connection, a practical innovation that can enable virtually any device to work with Welch Allyn FlexiPort Blood Pressure Cuffs - without the hassle of traditional cuff tubes and blood pressure connectors.

How Can FlexiPort Cuffs Help You?
1. Enhance Patient Safety
FlexiPort Cuffs can be used with virtually any blood pressure device, making it easier to find the proper-sized cuff for your patient to help prevent miscuffing.
2. Increase Staff Satisfaction
The single-point FlexiPort connection can improve nursing workflows, delivering a more reliable connection system and fewer complaints from hospital staff
3. Improve the Bottom Line
FlexiPort Cuff standardization can help reduce excess inventory costs by up to $140k, lowering cuff inventory levels as much as 90%.
4. Reduce Risk
FlexiPort Cuff technology offers hospitals options that facilitate single patient use to help reduce the risk of cross-contamination.
Why Standardise with FlexiPort?
- Simplifies purchasing and stock management
- Helps improve blood pressure and trend accuracy
- Reduce cuff faults and machine downtime
- Helps save valuable nursing time
- Eliminate cuff and machine mismatches
- More cuff options than other providers
In a hospital survey 100% of staff agreed that FlexiPort had made their working lives easier

A newly standardised hospital estimates that spending on cuffs will reduce by 75%
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | No |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |

Welch-Allyn #REUSE-11-2MF, FlexiPort Blood Pressure Cuff; Size-11 Adult, Reusable, 2-Tubes (8.0 and 13.0 in/20.3 and 33.0 cm), Mated Locking/Subminiature (#5082-182 and #5082-178)
$57.13 EACH

Welch-Allyn #REUSE-13-2MF, FlexiPort Blood Pressure Cuff; Size-13 Thigh, Reusable, 2-Tubes (8.0 and 13.0 in/20.3 and 33.0 cm), Mated Locking/Subminiature (#5082-182 and #5082-178)
$88.43 EACH

Welch-Allyn #REUSE-11L-2MF, FlexiPort Blood Pressure Cuff; Size-11L Adult Long, Reusable, 2-Tubes (8.0 and 13.0 in/20.3 and 33.0 cm), Mated Locking/ Subminiature (#5082-182 and #5082-178)
$70.70 EACH

Welch-Allyn #REUSE-12-2MF, FlexiPort Blood Pressure Cuff; Size-12 Large Adult, Reusable, 2-Tubes (8.0 and 13.0 in/20.3 and 33.0 cm), Mated Locking/ Subminiature (#5082-182 and #5082-178)
$43.93 EACH