-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

3M 90502 - DRESSING, TEGADERM, AG MESH, SILVER, 4", EACH
3M Tegaderm Ag Mesh Dressing with Silver 90502, 4 in. x 8 in. (10 cm x 20 cm) , 5 Dressings/ Box, 4 Boxes/ Case
3M Tegaderm Ag Mesh Dressing with Silver provides a fast-acting, long-lasting antimicrobial barrier in an affordable dressing
3M Tegaderm Ag Mesh Dressing with Silver is a nonwoven gauze dressing that contains silver sulfate, 8 mg/g of dressing. Silver sulfate released as silver ions within the dressing creates an effective barrier for up to 7 days. The soft, absorbent dressing is supplied sterile and may be custom cut. The porous, non-occlusive dressing conforms to the wound base and wicks drainage into the dressing where the silver ions are available to reduce the number of bacteria and yeast that are absorbed into the dressing.
- Easy to use, can be cut, folded or fluffed
- Effective up to seven days
- Effective against a wide range of microbes, including yeast, fungi and antibiotic-resistant bacteria (MRSA and VRE)
- Versatile, may be used as primary dressing or with absorbent wound fillers
- Can be moistened with wound exudate, sterile normal saline, sterile water or liquid hydrogel
- Ag Mesh Dressing with Silver
- Fast-acting, long-lasting antimicrobial barrier in an affordable dressing
Indications
Under the supervision of a health care professional, this dressing may be used as a primary wound dressing over:
- abrasions
- ulcers
- trauma wounds
- surgical wounds
- first and second degree burns
- donor sites
Contraindications
Tegaderm Ag Mesh Dressing with Silver should not be used on individuals that have a known hypersensitivity to silver or cotton. This product is not indicated for use as a surgical sponge or for use on third degree burns. This product should not be used with enzymatic debriding agents that are contraindicated for use with silver products.
The Proven Effectiveness of Antimicrobial Cationic Silver in an Affordable Gauze Dressing
Dressing Construction | Easy. Versatile. Affordable.
Silver sulfate is one of the most soluble silver salts
Dissolves in the presence of moisture releasing antimicrobial cationic silver
Cotton non-woven gauze
- Material that clinicians know and trust
- Can be cut to desired shape and size
- Can be fluffed or packed into tunnels, sinus tracts and areas of undermining
- Soft and conformable
Moisture Options
Can be moistened with wound exudate, sterile normal saline, sterile water or hydrogel. Sterile normal saline does not cause a significant change in pH, unlike some competitive products.
Why use 3M Tegaderm Ag Mesh Dressing with Silver?
Tegaderm Ag Mesh dressing meets the key performance criteria identified by clinicians:

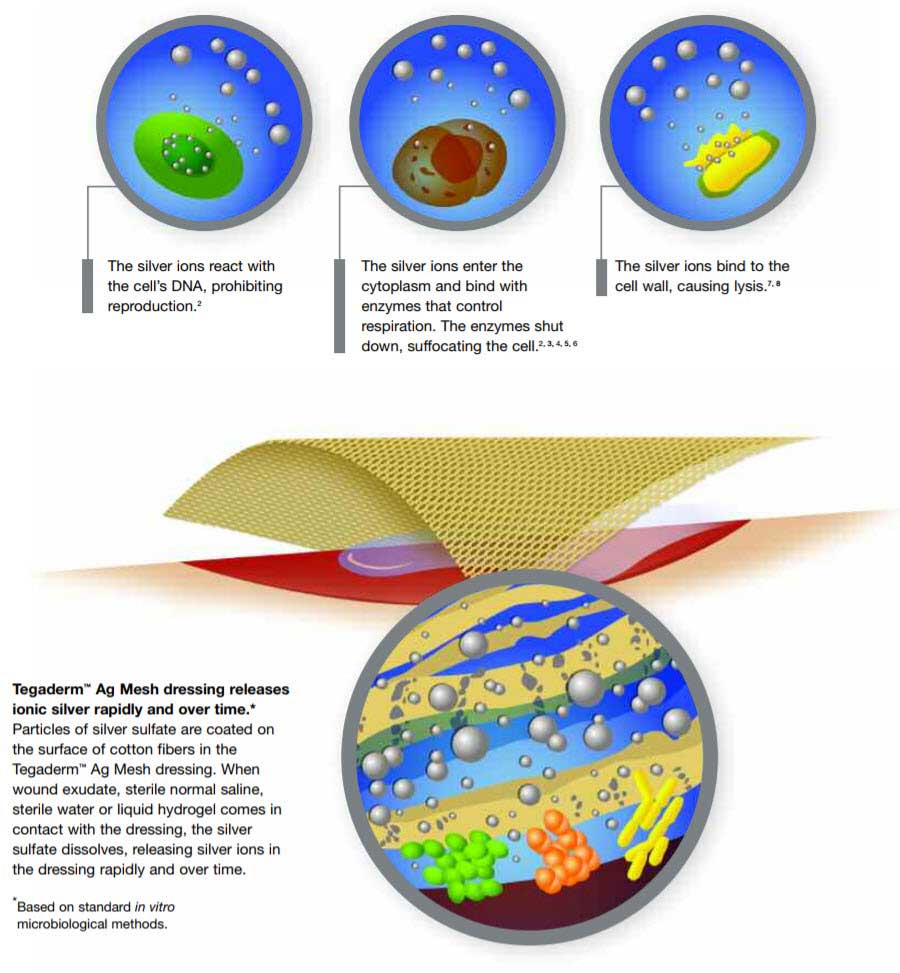
Ionic Silver Works 3 Ways to Control Bacteria
Clinical literature shows silver ions reduce microbial load through multiple mechanisms of action. This literature suggests that the risk of microorganisms becoming resistant to ionic silver is minimized because of silvers ability to destroy microbes in these ways:
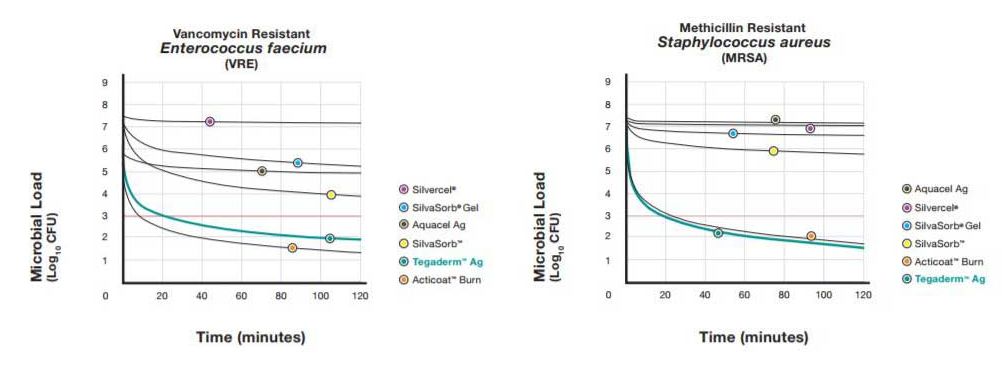
Fast-Acting against the Most Challenging Pathogens
Pathogens can multiply rapidly in a wound, so quick microbe reduction is important to wound management. Based on standard laboratory time-kill assays, Tegaderm Ag Mesh dressing reduced the number of pathogens including common, drug-resistant bacteria rapidly over 2 hours. In statistical comparisons, the performance of Tegaderm Ag Mesh dressing at 120 minutes was comparable to that of the leading fast-acting silver dressing, and exceeded the performance of several other silver dressings.

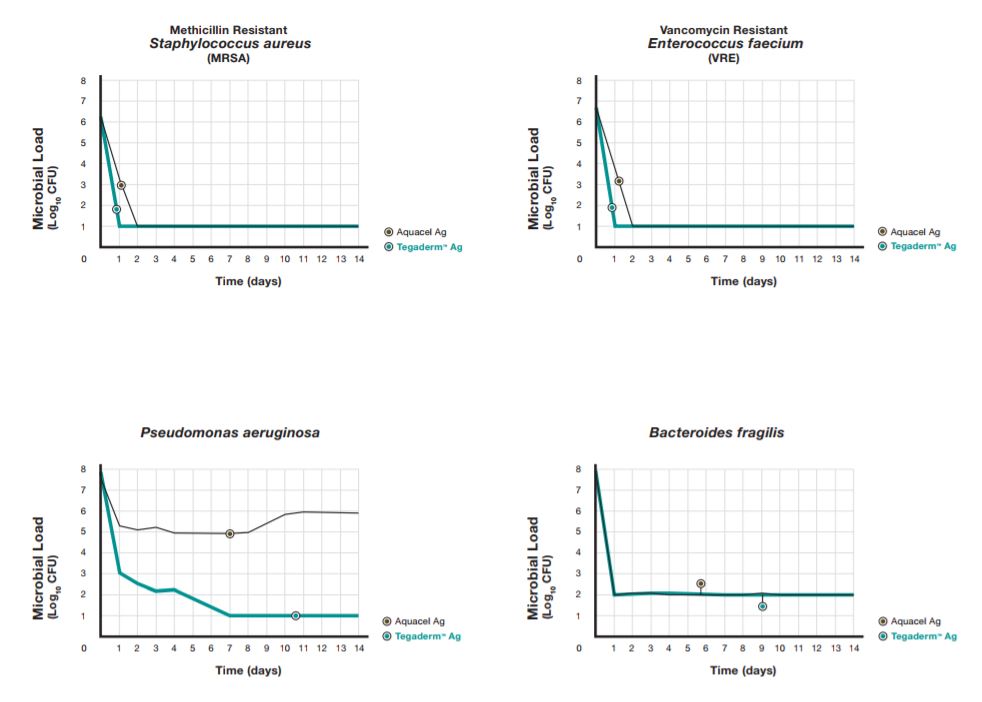
Long-Term Microbial Suppression for Added Effectiveness
Long-term suppression of wound microbes minimizes pathogen regrowth and possible interference with wound healing.14 Based on an in vitro time-kill assay with longer exposure times, Tegaderm Ag Mesh dressing demonstrated extended effectiveness with sustained microbial reduction over 14 days. The performance was comparable to that of a leading long-lasting silver dressing.
Efficacy in Controlling Varied Wound Flora
The following in vitro zone of inhibition studies 15 demonstrate the effectiveness of Tegaderm Ag Mesh dressing in controlling varying populations of bacteriaboth from laboratory samples and from wound exudate isolates of patients with non-healing wounds.
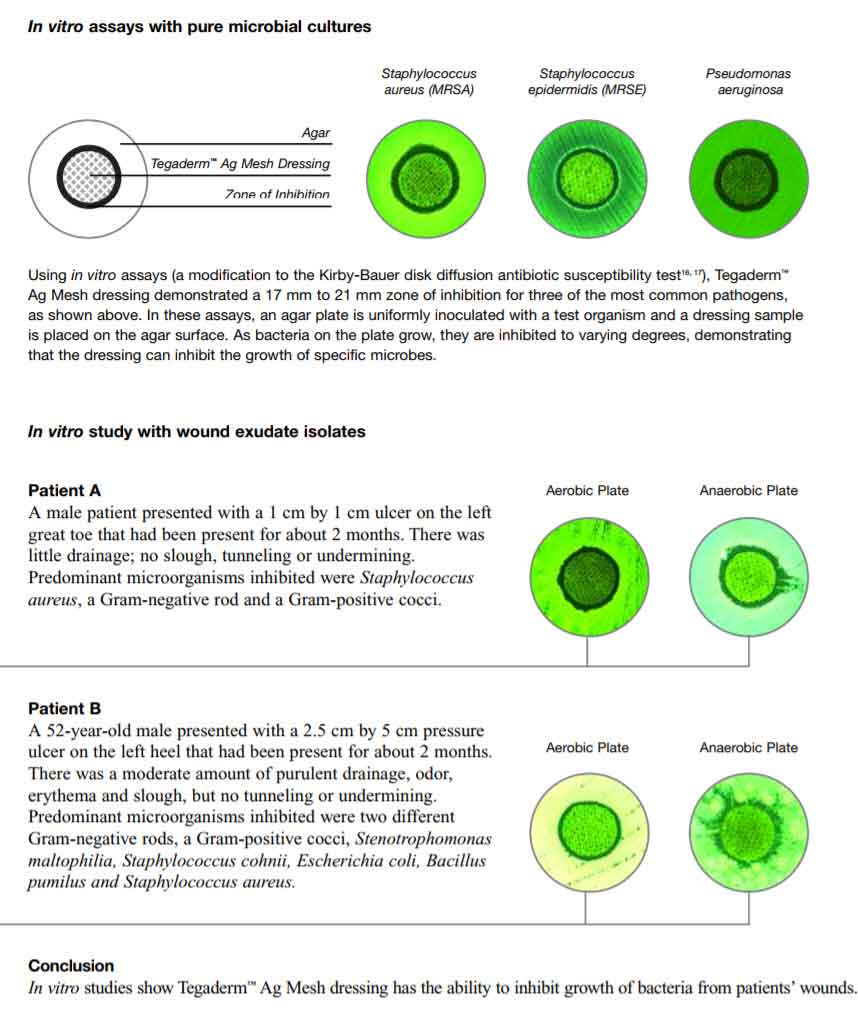
Dressing Application

Removing the Dressing
- Change the dressing as needed. Frequency of changing will depend on factors such as the type of wound and volume of drainage.
- At the time of dressing change, if the dressing is adhered to the wound surface, saturate with sterile normal saline or sterile water, allow the dressing to soften, and gently remove.
- Avoid forceful removal of the dressing to minimize disruption of the wound.
Precautions
- Tegaderm Ag Mesh Dressing with Silver must be moist to release ionic silver and minimize adherence to the wound. When used on dry or minimally draining wounds, the dressing should be moistened prior to application.
- It is possible that the amount of drainage could decrease rapidly during dressing use. Non-woven material may adhere to the wound or site if allowed to dry; this may result in disruption of the wound or site upon removal. When using the dressing, the health care professional may want to change the dressing more frequently to maintain moisture of the dressing and observe the condition of the wound or site.
- If the dressing has dried and is adhered to the wound surface, saturate with sterile normal saline or sterile water, allow the dressing to soften, and gently remove.
- Consult a health care professional if any signs of infection are noted. Signs of infection may include: pain, redness, swelling, unusual odor or drainage, absent or delayed healing, or fever. This dressing may be used on infected wounds only under the care of a health care professional.
- If the wound does not begin to show signs of healing or if any other unexpected symptoms occur, consult a health care professional.
- For external use.
- Remove the dressing prior to MRI (Magnetic Resonance Imaging) procedures.
- Avoid cutting the dressing into narrow strips; the dressing may be rolled for use in tunneled wounds which helps retain the tensile strength of the dressing.
- Clinicians/Healthcare Professionals should be aware that there are very limited data on prolonged and repeated use of silver containing dressings, particularly in children and neonates.
- Do not reuse. Reuse may result in compromising product integrity and lead to device failure.

3M #90501, DRESSING, TEGADERM, AG MESH, SILVER, 4"X5", EACH
$9.30 EACH

3M #90614, Tegaderm Foam Dressing 2.75x3 10/Bx, 4 BX/CA
$194.36 CASE

3M #90612, Dressing Tegaderm Foam Sq 5-5/8x5-5/8" Adhesive 10/Bx, 4 BX/CA
$269.88 CASE

3M #90611, DRESSING, FOAM, TEGADERM, OVAL, SM, 4"X4.5", EACH
$7.03 EACH

