-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

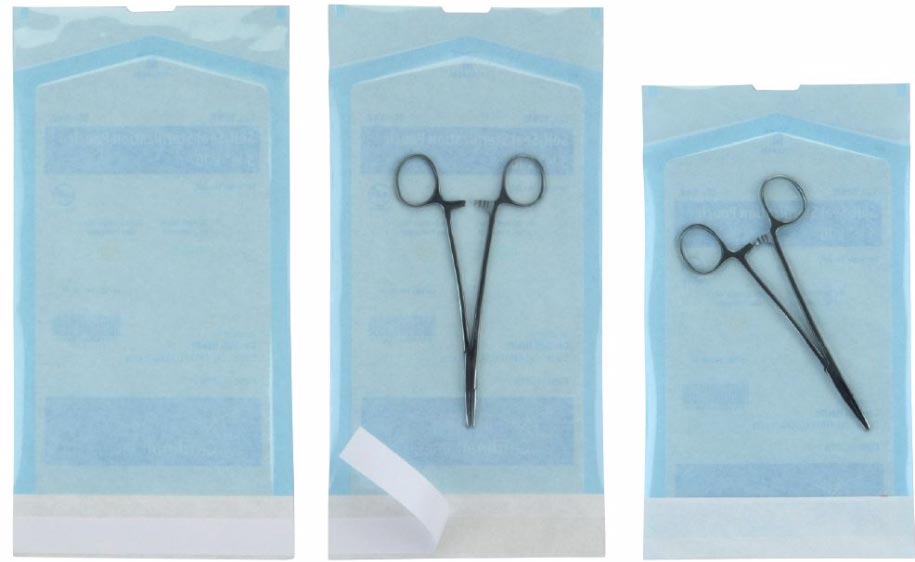
Cardinal Health 32218 - POUCH, STERI, SELF-SEAL, CLEAR, 12" X 18", 400 EA/CS, 4 PK/CS
Sterilization Peel Pouch
Sterilization Pouch combines a blue film and a medical grade TYVEK to provide breathability, sterile barrier, and indication of processing. Each pouch includes an external process indicator for Steam and Ethylene Oxide (EO) Gas sterilization.
Low Temperature Sterilization Pouch
Low Temperature Sterilization Pouch is an excellent choice for devices requiring low-temperature sterilization.
Low Temperature Sterilization Pouch Features
- Size 12 x 18 in.
- The pouch is made from a breathable, TYVEK material and a clear film.
- It includes an external process indicator for Ethylene Oxide (EO) gas sterilization.
- A convenient thumb notch at the top of the pouch to aid opening.
- The self-seal style includes a pre-folded bottom flap with an adhesive strip for convenience.
Packaging
Packaging is an important step in the sterile processing of reusable medical devices.
Sterilization packaging must-
- Allow sterilization of enclosed devices.
- Maintain sterility until opened.
- Provide for aseptic delivery of contents onto sterile field.
Maintanance
- Maintenance or dust covers are protective plastic bags used to help maintain the sterility of an item by protecting it from the environment.
Indications for Use
Low Temperature Sterilization Pouch and Tubing is intended to be used to enclose another medical device, in a single or double pouch configuration, that is to be sterilized by using,
- Ethylene Oxide (EO) with a concentration of 735 mg/L at 55C (130F) and 50% to 80% relative humidity for 60 minutes.
- The pouches are intended to allow sterilization of the enclosed medical device(s) and also to maintain sterility (SAL=10-6).
- The subject device is intended and has been validated to maintain sterility of the enclosed devices for 6 months after steam sterilization and 24 months after EO sterilization.
- The maximum validated pouch load is 2.64 pounds (1.2kg).
- These products are to be used for medical products without lumens.
Recommendations for EtO sterilization cycle

After EO sterilization, the color of external indicator will change from yellow to brown

Cardinal Health #92152, POUCH, STERI, SELF-SEAL, PEEL, 12" X 15", 400 EA/CS, 4 PK/CS
$45.89 per BOX

Cardinal Health #92152, POUCH, STERI, SELF-SEAL, PEEL, 12" X 15", 400 EA/CS, 4 PK/CS
$387.24 per CASE

Cardinal Health #92152, POUCH, STERI, SELF-SEAL, PEEL, 12" X 15", 400 EA/CS, 4 PK/CS
$387.24 per CASE

