-
Catheters (6,800+)
- Angiocatheters (50+)
- Closed System Catheters (300+)
- External Catheters (620+)
- Hydrophilic Catheters (140+)
- IV Catheters (1,200+)
- Non-Hydrophilic (20+)
- Plastic Catheters (200+)
- Rubber Catheters (700+)
- Silicone Catheters (770+)
- Ureteral Catheters (100+)
- Urethral Catheters (450+)
- Venous Catheters (240+)
-
Coronavirus (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (20,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Custom Kits
- Dental (14,000+)
- Gloves (8,000+)
-
Gynecology & Urology (1,000+)
- Bed Side Drainage Bags (350+)
- Circumcision (150+)
- Cord Clamps and Clippers (60+)
- Disposable Vaginal Specula (60+)
- Enema Bags (30+)
- External Catheters (620+)
- Foley Catheters and Trays (1,200+)
- Identification (1100+)
- Leg Bag Accessories (10+)
- Leg Bags (280+)
- Reusable Vaginal Specula (900+)
- Specimen Collection (200+)
- Tubing & Connectors (17,000+)
- Urinals / Bed Pans (1,300+)
- Urine Collectors (60+)
- Urological Irrigation Products (10+)
- Vaginal Specula Illumination (2+)
- Systems (11,000+)
- Hygiene (1,000+)
- Incontinence (1,000+)
-
Infection Control (2,500+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Iodine (460+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Infusion All (2,000+)
- IV Bags - Empty (300+)
- IV Bags - Filled (100+)
- Masks (3,800+)
-
Medical Apparel (23,000+)
- Arm Sleeves (240+)
- Beard Covers (20+)
- Bouffant Caps (200+)
- Compression Socks (80+)
- Coveralls (500+)
- Disposables (100+)
- Isolation Gowns (360+)
- Lab Coats (2,200+)
- Lab Jackets (300+)
- Patient Gowns (300+)
- Procedural Gowns (230+)
- Scrubs (20,000+)
- Shoe Covers (270+)
- Surgeon Caps (40+)
- Surgical Gowns (70+)
- Surgical Hoods (20+)
- Surgical Masks (330+)
- Ostomy (400+)
-
PPE (20,000+)
- Bacterial Filters (170+)
- Bleach (360+)
- Coveralls (500+)
- Disinfectant Wipes (350+)
- Face Shields (200+)
- Gloves (8,000+)
- Gowns (2,300+)
- Isopropyl Alcohol (170+)
- IV Therapy (2,000+)
- Masks (3,700+)
- Pulse Oximeters (250+)
- Sanitizer (670+)
- Scrubs (23,000+)
- Soap (1,500+)
- Stethoscopes (700+)
- Thermometers (950+)
- Respiratory (500+)
- Sanitizer (600+)
- Surgical Supplies (14,000+)
- Sutures (7,500+)
- Syringes & Needles (14,000+)
-
Wound Care (5,000+)
- ABD Pads (100+)
- Adhesive Bandages (650+)
- Advanced Wound Care (400+)
- Applicators (6,700+)
- Burn care (240+)
- Dressings (7,500+)
- Elastic Bandages (1,600+)
- Gauze (3,300+)
- Ice / Heat Packs (280+)
- Medical Tape (820+)
- Non-Adhering Dressings (100+)
- Ointment & Solutions (450+)
- Self-Adherent Wraps (200+)
- Sponges (2,400+)
- Staple & Suture Removal (1,500+)
- Tegaderm (450+)
- Transparent Dressing (800+)
- Wound Care Prep (120+)
- Wound Cleansers (100+)
- Sales & Deals (100+)
- 3M (4,200+)
- Alaris Medical (600+)
- Amsino International (550+)
- Avanos Medical (40+)
- B Braun (1,500+)
- Baxter (750+)
- BD (2,800+)
- BSN Medical (2,000+)
- Cables & Sensors (3,200+)
- C.R. Bard (4,200+)
- Cardinal Health (6,800+)
- CareFusion (2,100+)
- ConMed (1,500+)
- Cook Medical (600+)
- Covidien (9,500+)
- DeRoyal (6,000+)
- Dukal (1,300+)
- Ethicon (4,100+)
- GE Healthcare (1,000+)
- Hartmann (600+)
- Hospira (530+)
- ICU Medical (1,700+)
- Masimo (170+)
- Medline (54,000+)
- Midmark (2,500+)
- Roche (300+)
- Smiths Medical (4,000+)
- Sunset Healthcare (450+)
- TrueCare Biomedix (20+)
- View All Brands (5,000+)

Welch-Allyn DS44-09 - Durashock Sphyg w/Cuff Grey Child Ea
Welch Allyn DuraShock DS44 Integrated Aneroid Sphygmomanometer; Gear Free, Shock Resistant, 5-Year Calibration Warranty; Inflation Bulb and Valve; Inflation Bulb and Valve; Size-09 Child, FlexiPort Reusable, 1-Tube Cuff
DuraShock is the first and only gear-free, shock-resistant aneroid sphygmomanometer technology. We expect you will receive years of trouble free operation from this product.
- Gear-free DuraShock technology
- Certified accuracy to +/- 3 mmHg
- Withstands a 30" drop, AAMI's standard shock resistant requirement
- Unsurpassed reliability with a unique integrated, cuff-mounted design
- Gauge rotates 360, for easy viewing from any angle
- Gauge snaps directly into cuff for quick cuff changes
- Premium inflation system, and lightweight for patient comfort
- Five-year calibration warranty
- Latex-free for safety
Components of the Welch Allyn DuraShock Integrated Aneroid Sphygmomanometer

Assembly Instructions
NOTE: To assemble the bumper to a Welch Allyn DuraShock integrated gauge (model DS44 only), gently slide bumper over the gauge until the bumper just contacts the crystal around the entire circumference. Do not force to the point where the bumper shape is deformed, as this will reduce the shock-resistant properties of the bumper. | 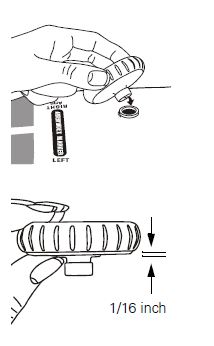 |
Operating Instructions
Auscultatory Method
- Select cuff size appropriate for the patients arm circumference. The applicable range, in centimeters, is printed on each cuff.
Note: The "Artery Index Marker on the cuff should fall within the "Range" indicated on the cuff. If the index marker falls short of range, a larger cuff should be used to ensure accurate results. If the index marker is past the range, a smaller cuff should be used to ensure accurate results. - Wrap the cuff around the arm with the artery index marker located over the brachial artery and with the lower edge of the cuff 1-inch (2.5 cm) above the bend in the elbow.
- Inflate cuff rapidly to a level 30 mm Hg above estimated (or palpatory) systolic pressure.
- Partially open the valve to allow deflation at a rate of 2 to 3 mm Hg per second.
- As the pressure falls, note systolic pressure and diastolic pressure with your stethoscope.
- Rapidly release the remaining pressure and record measurements immediately. After a minimum of 30 seconds, repeat the above steps for a second reading.
Inflation System Change and Replacement
The Welch Allyn DuraShock integrated blood pressure gauge connects effortlessly to the inflation system via a unique port connection. Therefore, only Welch Allyn integrated blood pressure cuffs can be used with the Welch Allyn DuraShock integrated blood pressure gauge.
- To remove the inflation system, simply grasp the gauge and pull away from the cuff. Rotating the gauge as it is being pulled away from the cuff will make removal easier.
- To attach a new or different size blood pressure cuff, simply press the gauge stem into the cuff port until you feel it engage. Rotating the gauge as it is being pressed into the cuff will make insertion easier.

Specifications and Standards
The Welch Allyn DuraShock integrated aneroid sphygmomanometer is accurate to 3 mm Hg and conforms to applicable sections of the following standards for aneroid sphygmomanometers:
- American National Standard ANSI/AAMI SP9-1994, Non-automated sphygmomanometers.
- European Standard EN 1060-1: 1996, Non-invasive sphygmomanometers, Part 1: General Requirements
- European Standard EN 1060-2: 1996, Part 2: Supplementary requirements for mechanical sphygmomanometers (excluding Section 7.4.3 for pointer thickness).
- INMETRO Technical Metrological Regulation Number 24 of February 26, 1996 (excluding Section 5.4 for pointer thickness).
This product will maintain the safety and performance characteristics specified at temperatures ranging from 0? (32?) to 46? (115?) at a relative humidity level not to exceed 85%.
How to Clean, Disinfect, and Sterilize the Welch Allyn DuraShock Sphygmomanometer
Cleaning
Aneroid Gauge, Inflation Bulb, and Valve: Clean the aneroid gauge, inflation bulb, and valve by wiping with slightly dampened cloth of alcohol pad.
Integrated One-Piece Cuff: Cuffs may be safely cleaned with a damp cloth (70% alcohol or 0.5% bleach solution may be used) or wash in warm water (140?, 60? maximum) with mild detergent. Before laundering the cuff:
- Remove the inflation bulb and valve.
- Close off the end of the tubing with Tube Plug Accessory (part no. 5082-163).
- Close off the cuff port with Cuff Port Laundering Plug (part no. 5082-250).
- Place the hook and loop fasteners in the closed position.
- Machine launder using gentle cycle, warm water, and mild detergent.
- Air dry completely and reassemble components.
DO NOT PRESS WITH HOT IRON.
Disinfecting
Glutaraldehyde-type liquid disinfectants may be used on the durable one-piece cuff. Follow instructions for use provided with the Glutaraldehyde product. Prolonged use of these disinfectants may cause discoloration. Do not use glutaraldehyde-type liquid disinfectants on the aneroid gauge, bulb, valve, or gladder.
Sterilizing
The durable integrated blood pressure cuff may be gas sterilized. Do not use steam or heat to sterilize the cuff, bulb, valve, or bladder. Do not attempt to sterilize the aneroid gauge.
Device Characteristics
| What MRI safety information does the labeling contain? | Labeling does not contain MRI Safety Information |
| Device required to be labeled as containing natural rubber latex or dry natural rubber (21 CFR 801.437): | No |
| Device labeled as "Not made with natural rubber latex": | No |
| For Single-Use: | No |
| Prescription Use (Rx): | Yes |
| Over the Counter (OTC): | No |
| Kit: | No |
| Combination Product: | No |
| Human Cell, Tissue or Cellular or Tissue-Based Product (HCT/P): | No |
Shipping Weights and Dimensions
| Gross Weight: | 0.316 KG |
| Width: | 7.62 CM |
| Height: | 20.32 CM |
| Depth: | 21.59 CM |





